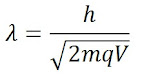Solution:
Given- potential
difference V= 200Volts
Where, q = charge on α-particle which is double of electronic charge = 2e= 2 x 1.6 x 10-19C = 3.2 x 10-19 C, h = Plank constant = 6.626x10-34Js
and m= mass of α-particle = 2(mass of proton)+ 2(mass of neutron)
m= 2x(1.67x10-27Kg) + 2x(1.67x10-27Kg)
m= 6.68x10-27Kg
Tags
quantummechanics